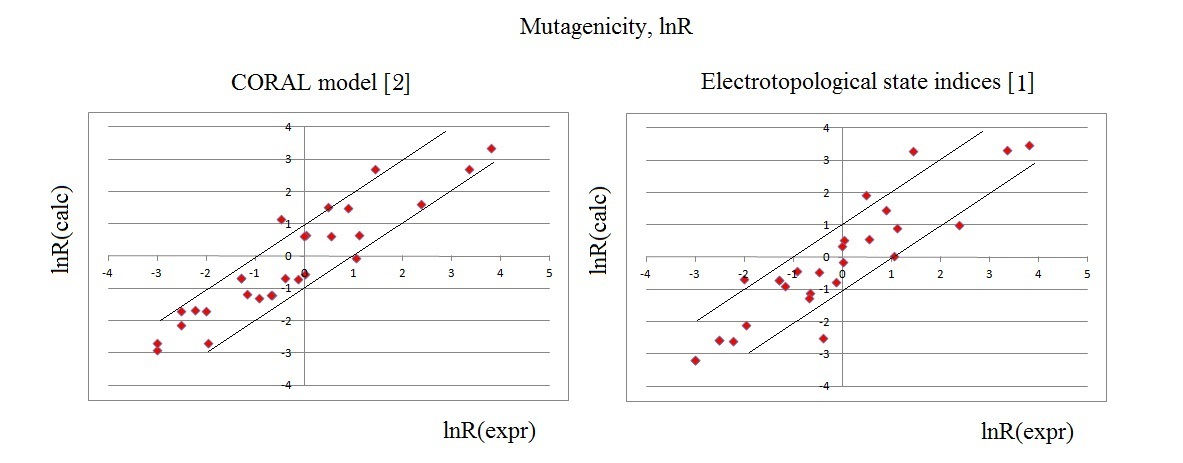
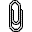

Mutagenicity
(revertrant/nmol, lnR): comparison of prediction by CORAL[2] and
described in
the literature [1] .
|
|
SMILES
|
lnR Expr |
CORAL[2]
Calc |
[1] Calc |
CAS No |
|
# |
Nc1cccc2ncccc12
|
-2.000 |
-1.696 |
-0.68 |
611-34-7 |
|
# |
Nc1ccc2ccccc2c1
|
-0.670 |
-1.206 |
-1.10 |
91-59-8 |
|
# |
Nc1cc2c3ccccc3Cc2cc1
|
0.890 |
1.484 |
1.46 |
6344-66-7 |
|
# |
Nc1ccc2c3ccc(N)cc3Cc2c1
|
0.480 |
1.528 |
1.92 |
525-64-4 |
|
# |
Nc1ccc(cc1)c2ccccc2
|
-0.140 |
-0.719 |
-0.76 |
92-67-1 |
|
# |
Nc1ccc(C)cc1OC
|
-1.960 |
-2.683 |
-2.12 |
16452-01-0 |
|
# |
Nc1ccc(cc1O)[N+]([O-])=O
|
-2.520 |
-2.131 |
-2.58 |
121-88-0 |
|
# |
Nc3cccc2c3ccc1ccccc12
|
2.380 |
1.595 |
0.99 |
4176-53-8 |
|
# |
Nc1ccc2nc3ccccc3nc2c1
|
0.550 |
0.614 |
0.57 |
2876-23-5 |
|
# |
CC(C)c1ccc(N)cc1N
|
-3.000 |
-2.902 |
-3.19 |
|
|
# |
Cc1cc(ccc1N)c2ccc(N)c(C)c2
|
0.010 |
-0.564 |
-0.14 |
119-93-7 |
|
# |
Nc4ccc3cccc2c1ccccc1c4c23
|
3.350 |
2.698 |
3.32 |
13177-25-8 |
|
# |
Nc1ccc(Cl)cc1
|
-2.520 |
-1.706 |
-2.58 |
106-47-8 |
|
# |
Nc1cc(ccc1)c2cc(N)ccc2
|
-1.300 |
-0.675 |
-0.71 |
2050-89-7 |
|
# |
Clc1cc(N)cc(Cl)c1N
|
-0.690 |
-1.214 |
-1.28 |
609-20-1 |
|
# |
Nc1cc2nc3cc(N)ccc3nc2cc1
|
1.120 |
0.658 |
0.89 |
7704-40-7 |
|
# |
Nc1ccc(OC)cc1C
|
-3.000 |
-2.683 |
-3.19 |
102-50-1 |
|
# |
Nc1cc(ccc1)c2ccccc2[N+]([O-])=O
|
-1.300 |
-0.696 |
-0.71 |
96187-18-7 |
|
# |
Nc2ccccc2c1ccc(N)cc1
|
-0.920 |
-1.315 |
-0.42 |
492-17-1 |
|
# |
Nc2cccc1nc3cccc(N)c3nc12
|
0.040 |
0.658 |
0.54 |
102877-14-5 |
|
# |
Nc3ccc4c2cccc1cccc(c12)c4c3
|
3.800 |
3.338 |
3.46 |
5869-25-0 |
|
# |
[O-][N+](=O)c1c2ccccc2ccc1N
|
-1.170 |
-1.183 |
-0.89 |
606-57-5 |
|
# |
Nc1ccc(cc1)c2ccc(N)cc2
|
-0.390 |
-0.675 |
-2.52 |
92-87-5 |
|
# |
Nc1ccc(cc1)c2ccc(cc2)[N+]([O-])=O
|
1.040 |
-0.056 |
0.02 |
1211-40-1 |
|
# |
Nc2cccc1nc3ccccc3nc12
|
-0.010 |
0.614 |
0.35 |
2876-22-4 |
|
# |
Nc1ccc(Cl)cc1[N+]([O-])=O
|
-2.220 |
-1.682 |
-2.61 |
89-63-4 |
|
# |
Nc1cc2c3ccccc3Nc2cc1
|
-0.480 |
1.152 |
-0.47 |
6377-12-4 |
|
# |
Nc4ccc1ccc2cccc3ccc4c1c23
|
1.430 |
2.698 |
3.29 |
1606-67-3 |