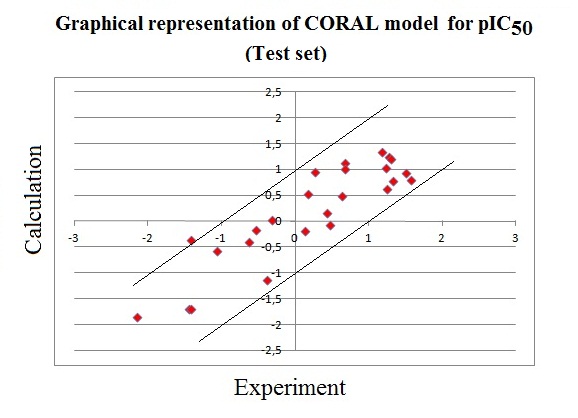
|
ID* |
Test
set |
Expr |
Coral [2] |
|
3 |
Cl.O=C(O)C3=CN(c1ccccn1)c2cc(c(F)cc2C3=O)N4CCC(N)C4
|
-1.433 |
-1.708 |
|
6 |
Cl.NC1CCN(C1)c2cc3N(C=C(C(=O)c3cc2F)C(=O)O)c4ccno4
|
-1.418 |
-1.703 |
|
10 |
Cl.NC1CCN(C1)c2cc3N(C=C(C(=O)c3cc2F)C(=O)O)c4nccs4
|
-1.410 |
-0.366 |
|
12 |
Cl.NC1CCN(C1)c2cc3N(C=C(C(=O)c3cc2F)C(=O)O)c4ncc(C)s4
|
0.175 |
0.525 |
|
14 |
Cl.NC1CCN(C1)c2cc3N(C=C(C(=O)c3cc2F)C(=O)O)c4nc(cs4)C(C)(C)C
|
-0.310 |
0.019 |
|
16 |
Cl.NC1CCN(C1)c2cc3N(C=C(C(=O)c3cc2F)C(=O)O)c4ncc(Cl)s4
|
0.639 |
0.481 |
|
24 |
Cl.NC1CCN(C1)c3c(F)cc4C(=O)C(=CN(c2nccs2)c4c3F)C(=O)O
|
-2.147 |
-1.852 |
|
32 |
Cl.CC1(N)CCN(C1)c2nc3N(C=C(C(=O)c3cc2F)C(=O)O)c4nccs4
|
1.175 |
1.342 |
|
37 |
Cl.N[C@H]1CN(C[C@@H]1OC)c2nc3N(C=C(C(=O)c3cc2F)C(=O)O)c4nccs4
|
1.246 |
0.617 |
|
40 |
Cl.OC[C@@]1(N)CCN(C1)c2nc3N(C=C(C(=O)c3cc2F)C(=O)O)c4nccs4
|
0.684 |
1.119 |
|
41 |
Cl.O=C(O)C4=CN(c1nccs1)c5nc(N2C[C@@H]3NCCO[C@H]3C2)c(F)cc5C4=O
|
-0.379 |
-1.132 |
|
42 |
Cl.O=C(O)C4=CN(c1nccs1)c5nc(N2C[C@@H]3CCCN[C@@H]3C2)c(F)cc5C4=O
|
0.136 |
-0.184 |
|
46 |
Cl.NC[C@H]1CN(C[C@H]1Cl)c2nc3N(C=C(C(=O)c3cc2F)C(=O)O)c4nccs4
|
0.434 |
0.164 |
|
50 |
Cl.NCC1CN(CCO1)c2nc3N(C=C(C(=O)c3cc2F)C(=O)O)c4nccs4
|
-0.630 |
-0.407 |
|
58 |
Cl.NC1CCN(C1)c2nc3N(C=C(C(=O)c3cc2Cl)C(=O)O)c4nccs4
|
-1.062 |
-0.587 |
|
63 |
Cl.CNC1CCN(C1)c3ccc4C(=O)C(=CN(c2nccs2)c4n3)C(=O)O
|
1.570 |
0.791 |
|
67 |
Cl.C[C@H]1CN(C[C@@H]1NC)c3ccc4C(=O)C(=CN(c2nccs2)c4n3)C(=O)O
|
1.507 |
0.927 |
|
73 |
Cl.N[C@H]1CN(C[C@@H]1OC)c3ccc4C(=O)C(=CN(c2nccs2)c4n3)C(=O)O
|
1.333 |
0.782 |
|
75 |
Cl.CN[C@@H]1CN(C[C@H]1OC)c3ccc4C(=O)C(=CN(c2nccs2)c4n3)C(=O)O
|
1.282 |
1.247 |
|
78 |
Cl.CN[C@H]1CN(C[C@H]1OC)c3ccc4C(=O)C(=CN(c2nccs2)c4n3)C(=O)O
|
1.303 |
1.192 |
|
81 |
Cl.C[C@H]1CN(C[C@@H](C)N1)c3ccc4C(=O)C(=CN(c2nccs2)c4n3)C(=O)O
|
0.678 |
1.002 |
|
88 |
Cl.O=C(O)C2=CN(c1nccs1)c3nc(C=C)ccc3C2=O
|
-0.524 |
-0.178 |
|
91 |
NC1CCN(C1)c2nc3N(C=C(C(=O)c3cc2F)C(=O)OCC)c4nccs4
|
0.472 |
-0.085 |
|
92 |
NC1CCN(C1)c2nc3N(C=CC(=O)c3cc2F)c4nccs4
|
0.277 |
0.944 |
|
94 |
NC1CCN(C1)c2nc3N(C=C(C=O)C(=O)c3cc2F)c4nccs4
|
1.229 |
1.019 |