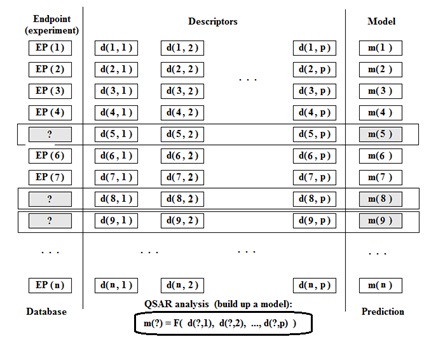
In other words QSAR paradigm for the traditional substances can be represented as the following:
Endpoint = F ( Molecular Structure )
The description and characterization of nanoparticles by traditional scheme is limited, because
(i) the molecular architecture of nanoparticles is extremely large and extremely complex;
(ii) there are specific interactions between different parts of the nanosystems which cannot be represented topologically
and / or by means of molecular mechanics and quantum mechanics.
The quantity turns into quality: the above paradigm for nanomaterials becomes unacceptable.
Under such circumstances, representation and characterization of nanomaterials should be organized by untraditional manner.
We have used the following principles of description and characterization of nanomaterials to build up model for endpoint related to nanomaterials.
1. Endpoint is any physicochemical or biochemical parameter of nanomaterials, that is (i) available for a group of similar nanomaterials; and (ii) that is useful information from practical point of view.
2. The paradigm of building up a model is the following:
Endpoint = F ( Available Information )
Available information can be
(i) data on chemical composition of a nanomaterial;
(ii) technological parameters related to the nanomaterial;
(iii) information related to synthesis of the nanometrial;
(iv) size;
(v) dose or concentration;
(vi) irradiation or dark;
(vii) dzetta potentials; etc.
In other words, we are using all available information on some group of nanomaterials.
3. A selected list of characteristics of nanomaterials should provide possibility to define the domain of applicability for a model. In other words, characteristics selected as the basis of description of nanomaterials should have satisfactory prevalence in both the training set and the test set.
Examples of the developed systems of characterization of nanomaterials are the following:
(i)Modeling of nanoparticles uptake in PaCa2 cancer cells [1];
(ii)Modeling of anti-HIV activity of fullerence derivatives [2].
Thus, we have suggested original principles of the description and characterization of nanomaterials.
References:
[1] Toropov A A, Toropova A P, Puzyn T, Benfenati E, Gini G, Leszczynska D, Leszczynksy J. QSAR as a random event: Models for nanoparticles uptake in PaCa2 cancer cells. Chemosphere 2013; 92 : 31-37.
[2] Toropov A A, Toropova A P, Raska I Jr, Benfenati E, Gini G. Development of QSAR models for predicting anti-HIV-1 activity using the Monte Carlo method . Central European Journal Chemistry 2013 ; 11 : 371-380.
A format for the representation of the NPs structure.
The representation of nanomaterials in standardized form is an object of research work. However, most probably the standardization will give clear transparent results (databases) in the future.
A possible way to organize a model for endpoints related to nanomaterials in the present time can be expressed in the form: endpoint is a mathematical function of all available eclectic information.
The eclectic information can be
(i)atom compositions;
(ii)conditions of synthesis;
(iii) the features of nanomaterials related to their manufacturing.
Naturally, the list can be easily extended (size, porosity, symmetry, electromechanical properties etc.).
In fact, the main task related to predictive modeling of nanomaterials can be defined as the following: the establishing of an optimal method to get a preferable results:
(i) quality of the nanomaterials;
and / or
(ii) list of perspective candidates for a technical and / or for a biochemical role, by means of the preliminary analysis of available experimental data including description of different ways of the performance of the experiment.
Thus, in order to define a predictive model for endpoint related to nanomaterials, one should exchange the paradigm of
Endpoint = F(molecular structure)
by the paradigm of
Endpoint = F(eclectic information)
In praxis, the approach can be demonstrated with model of thermal conductivity of micro-electronic-mechanic-systems (MEMS), where chemical composition, temperature, and technological attributes of MEMS are involved to build up the model.
The model has been calculated using developed by us the CORAL software (http://www.insilico.eu/coral). Table 1 shows the codes of technological attributes of MEMS.